New Silica-Based Adsorbent Developed for Selective Separation of Radioactive Strontium from Acidic Medium
Recently, Prof. HUANG Qunying’s team from Hefei Institutes of Physical Science (HFIPS) of Chinese Academy of Sciences (CAS) developed a novel inorganic silica-based adsorbent for the highly selective separation of strontium from acidic medium.
The related results were published in Separation and Purification Technology.
Radioactive strontium (90Sr) has been considered to be one of the most hazardous radionuclides due to its high biochemical toxicity. During the vitrification process of high level liquid waste (HLLW), the presence of 90Sr can cause instability of the vitrification substrate and thus induce radionuclides leaching. The removal of 90Sr can reduce the heat generation and shorten the cooling time of the vitrification substrate in the repository, which is favorable for further deep geological disposal of the radioactive waste.
To address the above issues, Prof. Huang’s team developed a novel silica-based adsorbent Sb2O5/SiO2 using a two-step method, i.e. vacuum impregnation followed by oxidation, and investigated the adsorption behavior of the adsorbent on strontium stable nuclide in low and high acid mediums (i.e. pH 6 and 1 M HNO3).
The experimental results showed that the prepared adsorbent possess good acid resistance stability and exhibit favorable adsorption on strontium stable nuclide in both low and high acid mediums. The mechanistic results revealed that the adsorption mechanism was ion exchange, and the adsorption was accompanied by the transfer of charge and the decrease of adsorption energy.
This study not only developed a novel method for the preparation of highly stable silica-based adsorbent, but also provided relevant experimental data and theoretical basis for the selective separation of strontium in acidic environments.
This work was supported by the National Natural Science Foundation of China.
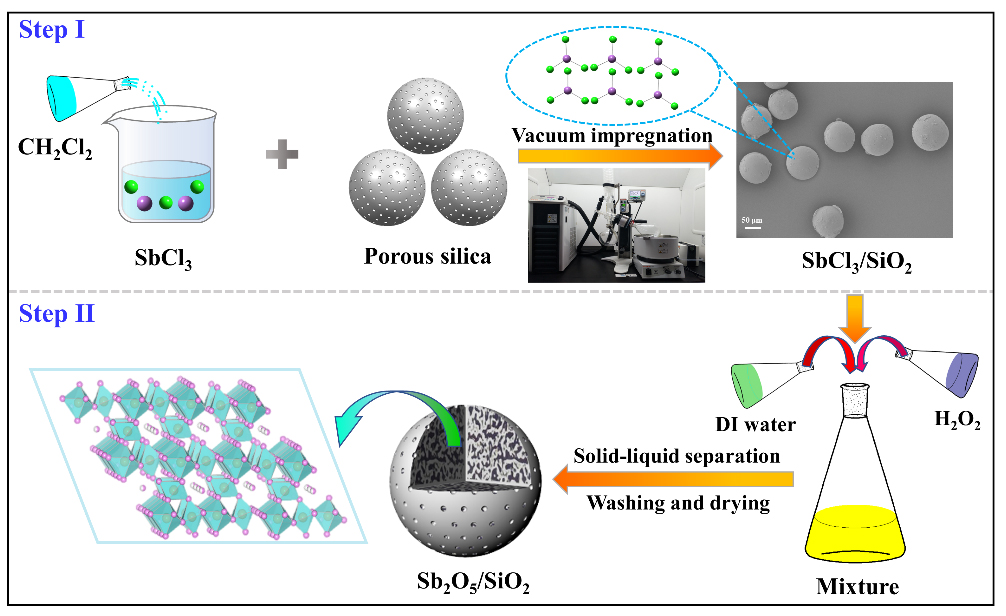
Schematic illustration of SbCl3/SiO2 and Sb2O5/SiO2 preparation processes. (Image by ZHANG Shichang)
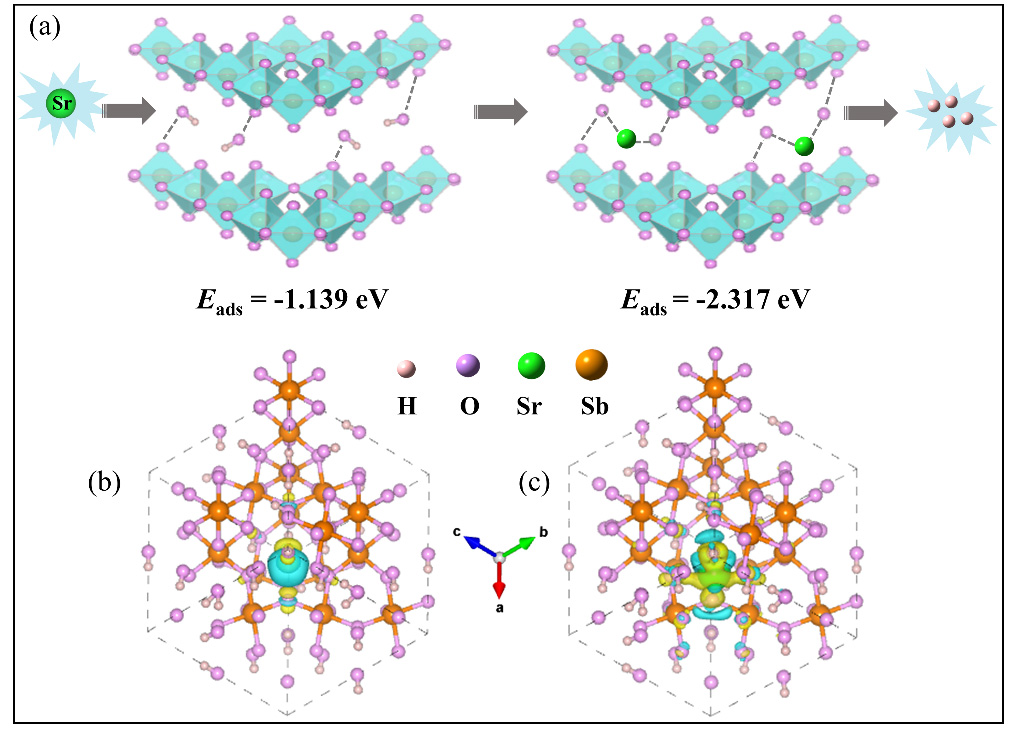
Adsorption mechanism and DFT calculations: (a) Schematic illustration of the adsorption mechanism; (b, c) Charge density difference of Sb2O5 before and after adsorbed Sr.(Image by ZHANG Shichang)














